Genobio takes the lead again ——The industry standard of "Bacterial Endotoxin Test Kit" is officially released
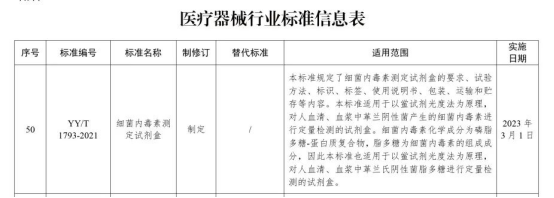
Following YY/T 1729-2020 "Fungi (1-3)-β-D glucan determination kit", YY/T 1793-2021 "Bacterial Endotoxin Determination Kit" formulated by Genobio will be released in 2021 On September 9, it was approved by the State Drug Administration and officially released. The standard will be formally implemented on March 1, 2023.

The preparation of the "Bacterial Endotoxin Test Kit" standard was organized by the National Medical Clinical Laboratory and In Vitro Diagnostic System Standardization Technical Committee (TC136), and it was officially launched in April 2019. With Genobio Pharmaceutical Co., Ltd. as the first drafter, united with Beijing Medical Device Inspection Institute, Beijing Medical Device Technology Evaluation Center, Shanghai Clinical Testing Center, Beijing Jinshanchuan Technology Development Co., Ltd. (a wholly-owned Subsidiaries) and many other units jointly drafted and formulated.
As a leading company in the domestic fungus/bacterial rapid inspection industry, Genobio is committed to the continuous upgrading of product standards. For more than 20 years, we have been guided by the industry's leading position and standardized market as our guide, constantly advancing with the times, striving for perfection, and continuously pursuing excellence. The promulgation of this standard can effectively standardize the quality of products in the industry and enhance the reputation of the bacterial endotoxin testing industry in the entire field of in vitro diagnostics.
Bacterial Endotoxin Detection Kit (Chromogenic method)
Genobio will continue to actively implement the standard publicity and implementation work, and advocate the improvement of industry product quality. At the same time, technical personnel will be organized to conduct standard publicity and implementation training for clinical and laboratory users in major hospitals, and "send standards to door".
In the future, Genobio will continue to utilize the industry leader's technical advantages, take the initiative to participate in the formulation of other related product standards, contribute its own strength to the standardization process of the in vitro diagnostic industry, and escort the safe development of my country's medical industry!


